Company FAQs
Our medicines initially target four diseases: multiple sclerosis, Parkinson’s disease, systemic sclerosis (a severe form of scleroderma) and Huntington’s disease. These diseases represent a combined $38 billion market and a huge unmet medical need, as there is no cure.
Multiple Sclerosis
Multiple sclerosis is an inflammatory and demyelinating disorder of the central nervous system affecting 2.3 million people worldwide. Myelin is a sheath around nerves that aids in conducting nerve impulses. Demyelination is a breakdown of this sheath. It is not reversible naturally or through existing drugs. As MS progresses, it causes pain affecting muscles, nerves, and joints, spasms, stiffness, and tremors, body mobility limitations and weakness, difficulty chewing or swallowing, in addition to speech difficulties.
Currently, there are several approved drugs on the market, all of which, are primarily for the relief of symptoms and none are disease-modifying or curative. Our preclinical data suggest that DHP-101 has the potential to be neuroprotective and disease-modifying, which could potentially be a significant advantage in the market.
Market value: US $16.3B in 2016; expected to reach US $27.38B by 2025.
Parkinson’s Disease (PD)
Parkinson’s disease is an incurable neurodegenerative disorder affecting nearly 10 million people worldwide. Primarily, it is a disease where the nerve cells stop producing a substance called dopamine, which helps transmit impulses from the brain to the muscles. It results in tremors, slowness and stiffness, impaired balance, and rigidity of the muscles. These symptoms get worse over time.
Currently, there is no cure for Parkinson’s disease, however, preclinical data show the potential of our drug, DHP-102, to provide neuroprotection and antiinflammation to improve clinical outcomes. Additionally, our treatment has shown the potential to reduce the loss of production of dopamine, the main issue with Parkinson’s disease.
Market value: US $2.15B in 2016; expected to reach US $5.24B by 2025.
Scleroderma (Systemic Sclerosis or SSc)
Scleroderma is an autoimmune disease that causes fibrosis, or thickening and scarring, of skin and internal organs. There is currently no cure and it is classified as an orphan disease in the US and EU. It is classified as an orphan disease (under 200,000 patients in a jurisdiction) in the US and EU.
Currently, there is no cure for scleroderma and approved therapies on the market today only address symptoms and are highly toxic. Our preclinical data in scleroderma suggests that DHP-101 may positively affect multiple anti-fibrotic pathways. Additionally, DHP-101 has been granted Orphan Drug Designation by the FDA in the United Kingdom and the EMA in Europe.
Market value: US $1.68B in 2017; expected to reach US $3.66B by 2024.
Huntington’s Disease (HD)
Huntington’s disease is an inherited disease that causes the progressive breakdown of nerve cells. It deteriorates patients’ physical and mental abilities to the extent that they are unable to care for themselves. It has no cure. In the U.S., approximately 30,000 people are symptomatic and more than 200,000 are at risk of inheriting the disease.
Currently, there are no drugs to slow or stop the progression of Huntington’s disease. There are specific drugs available to reduce some of the symptoms. Our preclinical data suggest that the neuroprotective potential of DHP-102 may provide a significant opportunity in the market. Additionally, DHP-102 has been granted Orphan Drug Designation by the FDA in the United Kingdom and EMA in Europe.
Market value: US $252.6M in 2014; expected to reach US $2.6B by 2024.
Our technology has been well-received by the scientific community and published in many independent, credible peer-reviewed scientific journals. You will find links to several publications that review the advances we’ve made in the development of new chemical entities for the treatment of neurodegenerative, autoimmune and other diseases here.
Oamaera Pharmaceuticals is a clinical development stage company. Our Phase IIa clinical trial of DHP-101 in systemic sclerosis patients (a severe form of scleroderma) is currently underway in the United Kingdom, Australia and New Zealand. Below you will find an image of our clinical development pipeline for your reference:
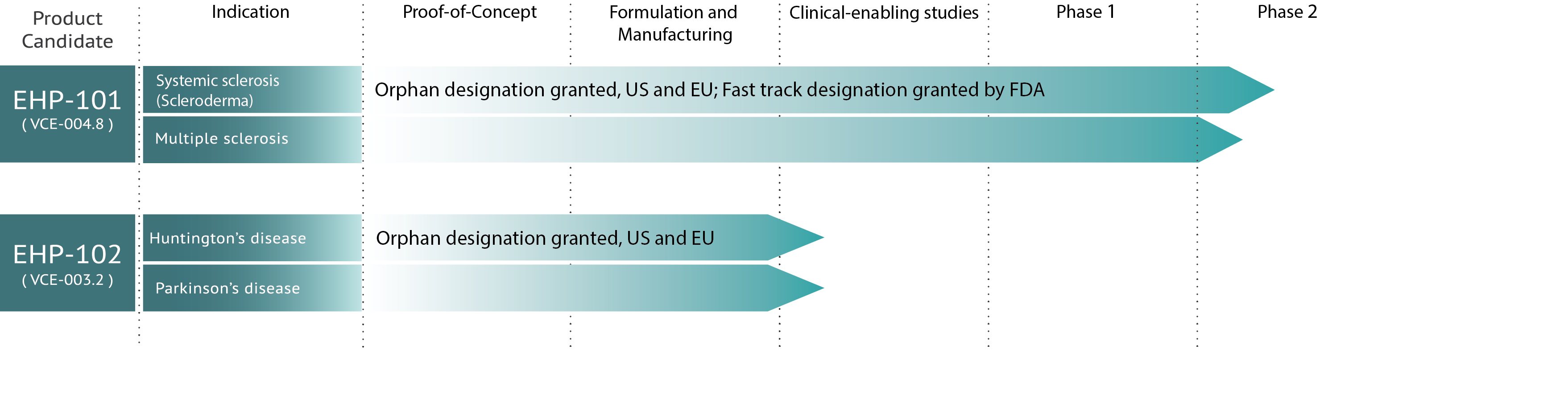
DHP has an international Phase IIa clinical trial of DHP-101 underway for systemic sclerosis and is in the process of initiating a Phase II international clinical trial of DHP-101 for multiple sclerosis.
Enrollment is open for our Phase IIa clinical trial focused on systemic sclerosis with study locations in the United Kingdom, Australia and New Zealand. For a list of participating research clinic locations, eligibility criteria and additional study details, visit www.ClinicalTrials.gov. It is important to speak directly with your doctor to determine your eligibility.
Enrollment for our Phase IIa international clinical trial of DHP-101 for the treatment of multiple sclerosis is expected to begin in early 2022.
Oamaera Pharmaceuticals is making great strides in advancing its mission to develop new therapies to tackle disease progression in various complex diseases which currently have no cure. If you would like to stay up-to-date on all future company developments, please sign up for our newsletter here.

